Collaborative research led by Yongsheng Zhou, Fuchou Tang and Jie Qiao on identification of human fetal bone marrow-derived stem cells at single-cell resolution
A fundamental question in the study of MSCs is that the bona fide identity of human MSCs in vivo is not well defined. In the past few decades, considerable progress has been made in defining and characterizing murine bone marrow MSCs using genetically engineered mice; and single cell RNA-seq technology helped us to gain a global recognition of the heterogeneous populations in the murine bone marrow (BM), however, the cell census of human fetal BM remains underestimated owing to material limitations.
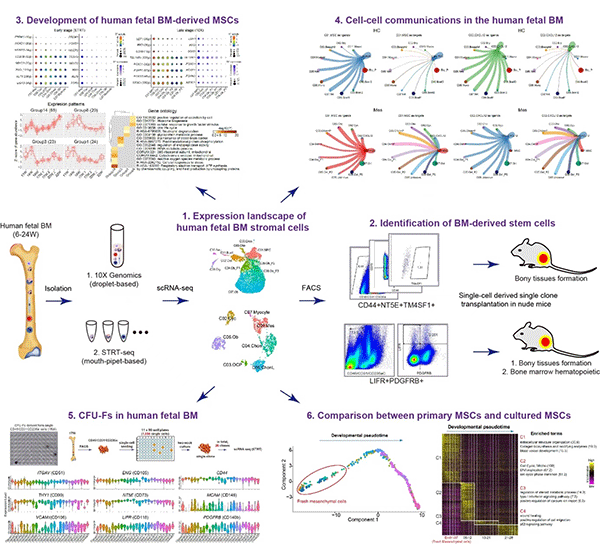
Recently, the team published a research paper titled "Characteristics of Dental Stem Cells in Human Fetal Bone Marrow by Single Cell Transcriptive and Functional Analysis" in the "Signal Transmission and Targeted Therapy (STTT)", we reported the expression landscape of human fetal BM nucleated cells (BMNCs) based on the single-cell transcriptomic analysis. While the common cell surface markers such as CD146, CD271 and PDGFRa used for isolating MSCs were not detected, LIFR+PDGFRB+ were identified to be specific markers of MSCs as the early progenitors. In vivo transplantation demonstrated that LIFR+PDGFRB+CD45-CD31-CD235a- MSCs could form bone tissues and reconstitute the hematopoietic microenvironment (HME) effectively in vivo. Interestingly, we also identified a subpopulation of bone unipotent progenitor expressing TM4SF1+CD44+CD73+CD45-CD31-CD235a- which had osteogenic potentials, but could not reconstitute HME. Stromal cells with CFU-F activity and multi-lineage differentiation ability in vitro were traditionally identified as MSCs. We found that many bone marrow stromal cells have the ability of multi-lineage differentiation potential and CFU-F activity in vitro, but failed to form new bone when transplanted in vivo. On the other hand, the expression pattern of many surface markers which are generally used for sorting MSCs displayed a great difference before and after culture. Our study suggested that it is not objective to identify MSCs based on CFU-F activity and trilineage differentiation in vitro.
Another critical question about the MSC-based bone tissue engineering is the necessity to expand the cells prior to use. In the current study, gene expression profiles indicated an apparent gap between fresh MSCs and cultured MSCs. The explicit alteration of fresh cells following ex vivo expansion, as well as the demonstration of in vivo niche for the MSCs in this work, is critical for guiding us to identify optimal culture conditions for maintaining the original characteristics of in vivo MSCs. This work not only expands our understanding of human bone marrow MSCs, but also provides great hints for further expansion of primary MSCs in vitro, which would be great help for MSCs-based bone regeneration.
Ping Zhang, associate professor of the Peking University School and Hospital of Stomatology, Ji Dong and Xiaoying Fan of Peking University, werre the co-first authors of the paper. Professor Yongsheng Zhou of the Peking University School and Hospital of Stomatology, Professor Fuchou Tang of Peking University and Academician Jie Qiao of Peking University Third Hospital were the co-corresponding authors. This study was funded by the National Natural Science Foundation of China and Peking University-Tsinghua University Joint Center for Life Sciences.
Link to the full text of the article: https://www.nature.com/articles/s41392-023-01338-2