Laboratory of Advanced Orthodontic Diagnostic and Therapeutic Strategies and Craniofacial Tissue Engineering
Our lab focuses on biomechanics and promoting strategies of orthodontic bone remodeling, digital diagnostic and treatment of malocclusion, development of advanced orthodontic appliances, craniofacial tissue engineering and research and translational application of biomaterials.
Mechanism and promoting strategies of orthodontic bone remodeling: Our research focuses on the mechanobiological mechanisms underlying alveolar bone remodeling. Under the stress-related microenvironment, our lab utilizes multi-omics technologies to identify the key cellular and molecular targets involved in alveolar bone remodeling. We aim to elucidate the cascade of mechanical tissue responses and clarify the biological mechanisms of bone metabolism regulated by orthodontic forces. Our work advances the frontier of bone biology, and provides a theoretical foundation and strategic insights for promoting efficient and safe alveolar bone remodeling.
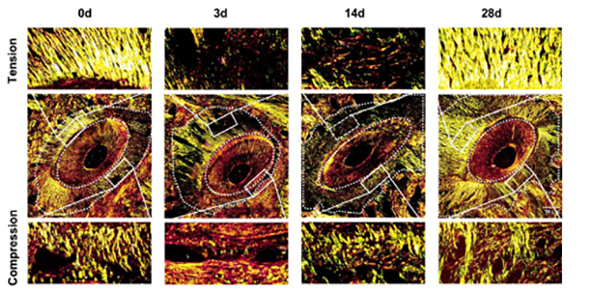
Research and development of intelligent system for diagnosis and treatment and efficient orthodontic appliances: Utilizing integration of multimodal data and AI-assisted analysis, our lab investigates the spatiotemporal patterns, safety thresholds, and relevant key determinants of orthodontic alveolar bone remodeling among patients with different skeletal types. Our goal is to define relative indications for orthodontic therapy and develop personalized, safe, and effective orthodontic strategies. We are also committed to develop advanced fixed-interwoven and clear aligner systems, supported by intelligent software for analysis, monitoring, and prediction, to enable smart and precise clinical treatment.
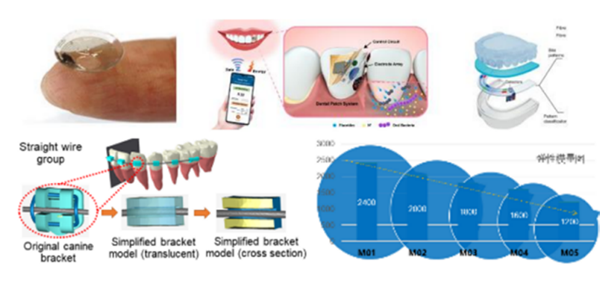

Craniofacial tissue engineering and research and translational application of biomaterials: Our team researches and develops innovative biomaterials for inflammatory bone and soft tissue defects in the oral and maxillofacial region. We utilize nanotechnology and protein engineering to fabricate biomimetic, multidimensional, biocompatible materials. These biomaterials enable spatiotemporally controlled and targeted delivery systems, precisely regulation of craniofacial stem cell fate, guidance of multicellular interactions, restoring host tissue homeostasis, and effectively promoting bone defect regeneration. Meanwhile, our team is developing anti-aging strategies to prevent and treat bone aging using smart, engineered nano-scaled vesicles. Engineered nano-scaled vesicles are functionalized with the ability to target at senescent stem cell, bone, or selectively clearing senescent cells. Cooperated with the intelligent controlled-release systems, these bioactive vesicles could efficiently achieve efficient intervention strategy in bone aging.
DIRECTOR

Bing Han, DDS, PhD
Professor
Principal Investigator
Kqbinghan@bjmu.edu.cn
Dr. Bing Han is a professor and Deputy Director of the Department of Orthodontics, Peking University School and Hospital of Stomatology. He is supported by National Special Support Plan for High-level Talents. He also serves as the Executive Director of the Tweed Center (China), the Executive Director of the Craniofacial Growth and Development Center, and a member of the standing committee of Chinese Orthodontic Society. Dr. Bing Han focusses on craniofacial growth and development, digital diagnosis and treatment of malocclusion, development of efficient orthodontic appliances, and development and clinical translation of oral-maxillofacial tissue engineering and biomaterials. He has led over 20 scientific research projects including National Natural Science Foundation of China, National Key Research and Development Program of China and Key Program of the Beijing Natural Science Foundation. He has published over 100 papers as the corresponding author about Dentistry, biomaterials and interdisciplinary research in Nature Biomedical Engineering, Nature Communications, Journal of Dental Research, Advanced Materials, Advanced Functional Materials, Bioactive Materials, Advanced Science, Prog. Orthod., Am. J. Orthod. Dentofac. Orthop., Angle Orthod., Orthod. Craniofac. Res., etc. He holds 43 authorized domestic and international patents and software copyrights. Dr. Bing Han has also received Second Prize of Beijing Award for Science and Technology Program, Second Prize of Chinese Stemmatological Science and Technology Prize, Peking University Distinguished Young Doctor Award and Chinese Medical Science and Technology Prize.
RESEARCH AREAS
Mechanisms and promoting strategies for orthodontic bone remodeling
This study focused on the response of the periodontal ligament between the alveolar bone and tooth root during orthodontic tooth movement. We traced the activation and differentiation of Lepr+ osteoprogenitors under orthodontic force. We found orthodontic force induced periodontal ligament cells to secrete apoptotic vesicles and activate osteoprogenitors, and this process requires a response period of 3–5 day for initiation. This study provides a theoretical basis for applying force in orthodontic clinical treatment, and lays the groundwork for further developing strategies of bone defect regeneration. The study was published in Journal of Dental Research (2024, 937,cover article) , International Journal of Oral Science (2024, 51), Clinical Oral Investigations (2021,5227) .
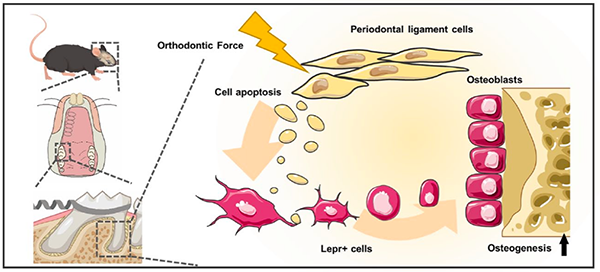
The diagram illustrates the experiment procedure of Lepr+ cells activated by PDLSC-derived apoptotic vesicles
Development of intelligent system for diagnosis and treatment and efficient orthodontic appliances
We proposed the theory of physiological anchorage and healthy orthodontic treatment. Our lab is developing safe and efficient orthodontic treatment system consisting of fixed and graded-property clear aligner systems. We also commit to develop a system capable of automatic diagnosis and treatment designs based on AI, which can enable the accurate prediction of orthodontic efficacy. The comparative study about anchorage control by this novel system was published in the American Journal of Orthodontics and Dentofacial Orthopedics (2025, 166), Angle Orthodontist(2024, 504), European Journal of Orthodontics (2020, 113) and Progress in Orthodontics (2025,2; 2024,2), which are top-tier journal in Orthodontics.
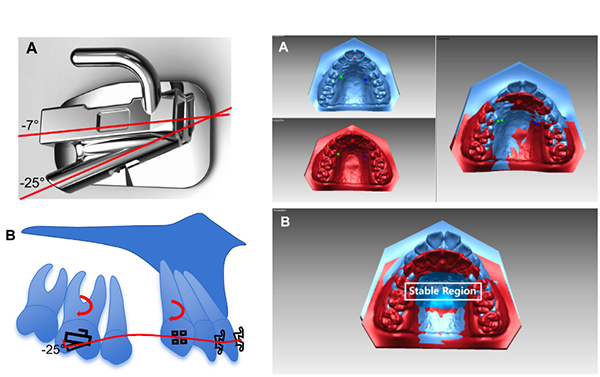
The structural diagram of XBT (Left);
Superimposition of pretreatment (blue) and posttreatment (red) maxillary 3D digital models (Right)
Craniofacial tissue engineering and translational biomaterials
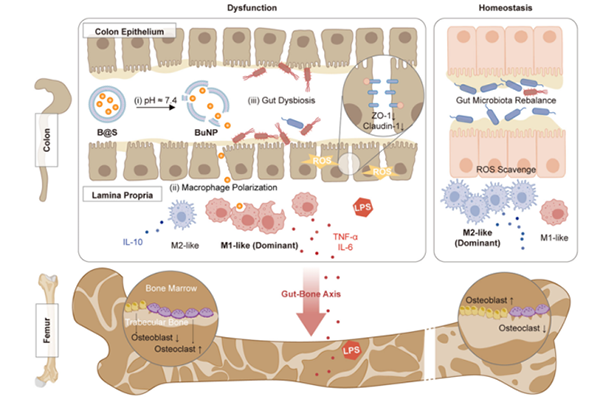
This study engineered postbiotics butyric acid by nanotechnology to construct postbiotics nanoparticles for treating colitis-induced secondary osteoporosis. Based on the gut-bone axis theory, we extended its application to treat primary osteoporosis and consistently demonstrated alleviation of bone loss and improvement in bone mass. The related results were published in Nature Communications (2024, 10893), Nature Biomedical Engineering (2024,215), Advanced Materials (2024, 2405164) , Bioactive Materials (2022, 130-139), Advanced Functional Materials (2024, 2402861).
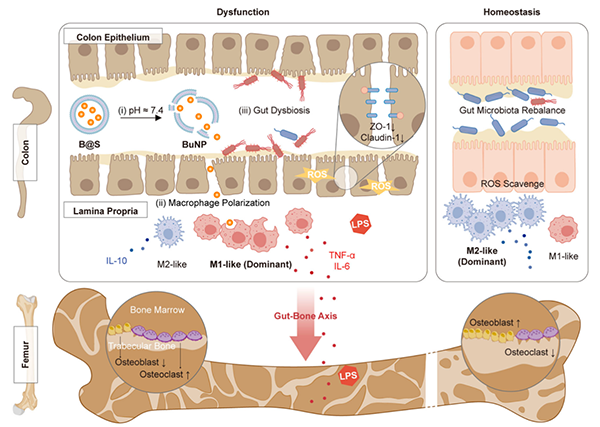
Schematic of engineered postbiotics nanoparticles in the regulation of gut-bone axis. The shellac shell protected the polyvinyl butyrate nanoparticles (BuNP), preventing degradation by gastric acid and facilitating the release of the BuNP in the lower region of the gastric intestinal tract at pH 7.4 (i). The nano-sized BuNP can then be internalized and hydrolyzed by macrophages, promoting anti-inflammatory polarity (ii). The sustained release of BuNP aids in rebalancing redox levels and gut microbiota in inflamed colon tissue (iii). Collectively, these mechanisms contribute to the efficacy of shellac-engineered postbiotics nano-particles (B@S) in treating gut-derived inflammation and post-menopausal-induced systemic bone issues.
RECENT PUBLICATIONS
Potent prophylactic cancer vaccines harnessing surface antigens shared by tumour cells and induced pluripotent stem cells
Nature Biomedical Engineering, 2024,9:215-233.
Colon-targeted Engineered Postbiotics Nanoparticles Alleviating Osteoporosis through Gut-Bone Axis
Nature Communications, 2024, 15:10893
Origin of ultrahigh-performance barium titanate-based piezoelectrics: Stannum induced intrinsic and extrinsic contributions
Nature Communications, 2024, 15:7700
Contribution of irreversible non-180o domain to performance for multiphase coexisted potassium sodium niobate ceramics
Nature Communications, 2024,15(1):2408
PDLC-apoptosis activates Lepr+ osteoprogenitors in orthodontics
Journal of Dental Research, 2024,103(9):937-947.
Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration
Bioactive Materials. 2022,11:130-139.
Age-related alveolar bone maladaptation in adult orthodontics: finding new ways out
International Journal of Oral Science,2024,16(1):51
Evaluating anchorage and torque control in Class II division 1 adolescent patients among three appliances
American Journal of Orthodontics and Dentofacial Orthopedics ,2025,167:166-176
Multi-functional Lanthanide Metallopolymer: Self-healing and Photo-stimuli-responsive Dual-emitting Luminescence for Diverse Applications
Advanced Materials, 2024, 36: 2405164.
Continuous “Snowing” Thermotherapeutic Graphene. Advanced Materials. 2020,32:2002024
Isolated-Oxygen-Vacancy Hardening in Lead-Free Piezoelectrics
Advanced Materials, 2022, 34(29): 2202558.
Mechanosensor YAP mediates bone remodeling via NF-κB p65 induced osteoclastogenesis during orthodontic tooth movement
Progress in Orthodontics, 2025,26: 2
Lysine 68 Methylation-dependent SOX9 Stability Control Modulates Chondrogenic Differentiation in Dental Pulp Stem Cells
Advanced Science, 2023,10: 2206757
Mechanically Robust Hydrogels Facilitating Bone Regeneration through Epigenetic Modulation
Advanced Science, 2022, 9: 2203734
Engineered extracellular vesicles from mesenchymal stem cells as a therapeutic strategy for bone-related diseases
Advanced Functional Materials,2024, 34: 2402861
Multi-Length Engineering of (K,Na)NbO3 Films for Lead-Free Piezoelectric Acoustic Sensors with High Sensitivity
Advanced Functional Materials,2023, 33: 2312699
Engineered bicomponent adhesives with instantaneous and superior adhesion performance for wound sealing and healing applications
Advanced Functional Materials. 2023, 33: 2303509.
Engineered fabrication of enamel-mimetic materials
Engineering. 2022, 14:113-123
MOF-Based Guided Bone Regeneration Membrane for Promoting Osteogenesis by Regulating Bone Microenvironment through Cascade Effects
Advanced Healthcare Materials,2024, 34: 2402861
Lower incisor position in skeletal Class III malocclusion patients: a comparative study of orthodontic camouflage and orthognathic surgery
Angle Orthodontist,2024,94(5):504
TEAM

Tingting Yu,DDS,PhD
Lab specialist
tingtingyu@bjmu.edu.cn

Yunfan Zhang, DDS, PhD
Lab specialist
YunfanZhang@bjmu.edu.cn

Si Chen, DDS,PhD
Clinical professor
elisa02@163.com

Xiaomo Liu, DDS,PhD
Clinical professor
momo96@163.com
CONTACT
Bing Han
Kqbinghan@bjmu.edu.cn
86-10-82195737
Department of Orthodontics,
Peking University School and Hospital of Stomatology,
No.22 Zhongguancun South Avenue,
Haidian District, Beijing 100081, PR China
last text: Weiran Li Lab
next text: Precision molecular diagnostics of oral and maxillofacial tumors






